Cu to Cu2+ Oxidation or Reduction
Cu Ag NO3- --- Ag Cu2 2NO3-Step 3. Cu2 2 E- Cu - Chemistry.
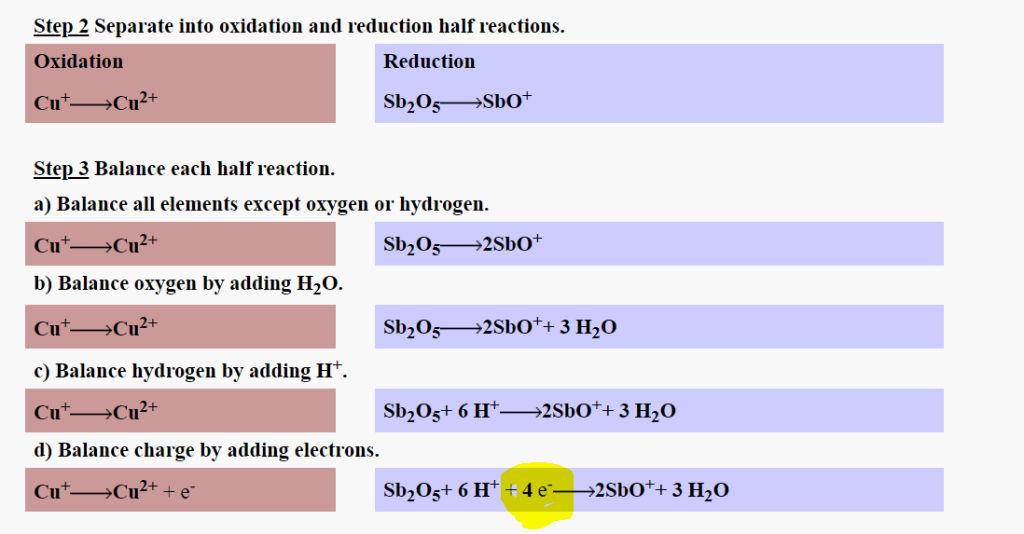
Solved Step 2 Separate Into Oxidation And Reduction Half Chegg Com
Cu2 2e- Cu Oxidation.

. Cu2 Fe Cu Fe2 111-3. The added electrons reduce the oxidation state of the substance. We can write two half reactions for this process one for Fe to Fe2 and the other for Cu2 to Cu.
2H2O O2- H 4e- Overall. This is an example of oxidation. You are taking two electrons out of that neutral copper atom to make a copperII cation.
A redox reaction is nothing but both oxidation and reduction reactions taking place simultaneously. For example Whereas in an oxidation-half reaction there is loss of electrons. Cu2 ions gain two electrons so they are reduced to Cu atoms.
This reaction takes place at the anode. Cu sCu2 aqAg aqAg s Anode oxidation Cu s Cu2 aq 2e-Eo ox -Eo Cu -0337 v Cathode reduction Ag e- Ag s Eo red E o Ag 0800 v 2 x Ag e- Ag s 0800 v Cu s Cu2 aq 2e -0337 v Cu s 2Ag aq Cu2 aq 2 Ag s 0463 v Eo cell Or. The conversion of Cu2 to Cu indicates.
Is Cu to Cu2 oxidation or reduction. 2 Fe2 Cu² Cu 2 Fe3 What is the standard potential Enet. The compound or the element that oxides are called reducted agent because it promotes the reduction of the other one.
If you have electrons in your equation then a reduction has then on the LHS and the oxidation has them on the RHS. Assign oxidation numbers to each species. The oxidation of double bonds in PE-containing bilayers can be monitored in the presence of Cu 2.
C Carbon tetrachloride is a non-electrolyte because it is a covalent compound. And identify what is being oxidized and what is being reduced. Professors will sometimes give you a mnemonic like LEO GER for loss of electrons is oxidation and gain of electrons.
Cu² aq 3NO₂ aq Cu⁰ aq 2NO₃ aq Explanation. Cu2 aq Zn sZn2 aq Cu s Experiment 2. Comment Clark Bull-Misner 2 years ago Follow The FORMAL LOSS of electrons specifies oxidation and we introduce oxidation numbers as labels to make electron lossgain specific.
Cu Cu 2 2e - R. 2Cu aqCu 2aqCus choose correct E o for above reaction if E Cu 2Cuo 034V and E Cu 2Cu o 015V A 038V B 049V C 038V D 019V Medium Solution Verified by Toppr Correct option is C From given data from ΔG onE oF. 2Cu2 2H2O 2Cu O2- H Cu2 is reduced because it gains 2 electrons in order to become Cu and reduction is the gain of electrons.
Copper is oxidized from 0 to 2 so Cu is the reducing agent. Similarly reduction could be considered as giving the electrons back or the removal of oxygen usually with. HNO 3 3 H 3e - NO 2 H 2 O Step 5.
OX Cu --- Cu2 RED Ag --- Ag. Eo cell EoCathode - EoAnode right left reduction. Cu aq is unstable in solution and undergoes simultaneous oxidation and reduction according to the reaction.
For example Thus we can conclude that the equation Cu cu2 2e represents oxidation-half reaction. The electrons lost in the oxidation half-reaction must be equal the electrons gained in the reduction half-reaction. Zinc reduced copper from Cu2 to Cu0so it is a stronger reducer than Copper.
Reduction is a gain of electrons. It does not ionize and hence do not conduct electricity. Cu2 2e - Cu b Just multiply oxidation half-reaction by 2 and reduction half-reaction by 1.
Strikingly it was found that the oxidation rate is 82 times faster at pH 74 for bilayers containing 70 mol PE than for pure phosphatidylcholine PC bilayers upon exposure of both to 70 μM Cu 2 and 10 mM hydrogen peroxide. State Whether the Following Conversion is Oxidation Or Reduction. Cu Ag NO3- --- Ag Cu2 2NO3-Cu is being oxidized and Ag is being reduced.
Mg --- Mg2 2e- oxidation and Cu2 2e- --- Cu reduction. The Molarity of Zn in the solution remains the same. This reaction takes place at the cathode.
Zinc replaced copper in the solution causing copper to precipitate as a solid. The compound or the element that reduces is called the oxidation agent for the same reason. Na - Na e.
Cu 2 I - Cu I 2 Step 2. B reduction by GAIN of electrons 2. D reduction by LOSS of electrons 2.
Copper is OXIDIZED from copper metal mathCu 0 math to cupric ion mathCu 2 math ie. Oxidation is technically adding oxygen so since Cu has no oxygen and Cu could be CuO it is oxidation. In a reduction-half reaction there is gain of electrons.
Fe2 Fe3 e Reduction. Select all that apply 2 Cus Cu2aq 2 e This is an oxidation. The electrons provided by Mg cause the reduction of Cu2 so Mg is the reducing agent.
Is Cu to Cu2 oxidation or reduction. Standard Reduction Potentials. Oxidation and Reduction Reduction is a gain of electrons oxidation is a loss of electrons and electron transfer reactions are also called redox reactions.
This is a reduction. In the active enzyme Cu ions are reactive to O2 but in the inactive enzyme Cu can be oxidized only by oxidizing agents such as H2O2 or ferricyanide or by a slow intermolecular electron transfer from Cu 615 to the active enzyme. Separate the process into half reactions.
2Na - 2Na 2e. A The reaction takes place at anode. Which occurs in the half-reaction Cu2 2e CU.
Answers the answer Cu cu2 2e this equation represents oxydation half-reaction of Cu II Explanation. This is a reduction. Select all that apply 2 Cus Cu2aq 2 e This is an oxidation.
This is an oxidation-reduction reaction since the oxidation numbers changed. A Assign oxidation numbers for each atom in the equation. B Cu2 will discharge easily at cathode.
Please log in or register to add a comment. Cu2 2e - Cu c 2Na Cu2 - 2Na Cu 2e cancels each other Please be reminded this answer may not be correct. C oxidation by LOSS of electrons 2.
A oxidation by GAIN of electrons 2. Cu2 2 e Cu Net. Which of the following is true about the half reaction below.
These two half reactions are given below. Because oxidation can be considered as giving electrons to the oxygen oxidation can also be considered as an atom giving up electrons. Make electron gain equivalent to electron lost.
Answer 1 of 19. Eg when Mg displaces Cu from Cu2 the half reactions are. In both species of enzyme rapid reduction of Cu2 ions by substrate takes place.
Fe s Fe 2 aq 2e- Oxidation half reaction Cu2 aq 2e- Cu s Reduction half reaction. A redox reaction is a reaction that occurs with oxidation and a reduction.

Complete The Following Equations And State Whether They Are Oxidation Or Reduction Reaction Cu Tocu 2


Comments
Post a Comment